Pathogen Detection
PCR Assays
Campylobacter

BAX® System PCR Assays for Campylobacter
Campylobacter (C. jejuni, C. coli, C. lari) is one of the most difficult pathogens to detect with conventional cultural confirmations due to the stringent microaerophilic environment. Avoid the labor and time consumption involved with conventional methods by utilizing the BAX System Real-time Assay for Campylobacter coupled with Hygiena’s validation and application library to achieve accurate and rapid detection across various matrices.
Catalog Number | KIT2018
Targets | C. jejuni, C. coli and C. lari
PCR Time | Real-Time (70 min)
Certificates | AOAC-RI PTM, USDA-FSIS, and NordVal
- Catalog Number: KIT2018
- Targets: C. jejuni, C. coli and C. lari
- PCR Time: Real-Time (70 min)
- Certificates: AOAC-RI PTM, USDA-FSIS, and NordVal
Increased
Lab Productivity

Simplified
Workflow

Accurate
Results

24-Hour
Support

Dedicated
Applications Lab

BAX® System PCR Tablets
These convenient BAX® System PCR tablets contain all the reagents needed for fast and reliable testing results. Simply hydrate with the prepared sample and load the rack into the BAX® System instrument for automated analysis.
Most Validated Matrices
With decades of experience in food safety, working with hundreds of top food manufacturers across a broad array of product groups, we’ve established dozens of protocols for the most difficult matrices. Our world-class applications scientists have solved the problem, so you don’t have to.
Accurate Results
In the food & beverage industry, accurate results mean fewer re-tests, shorter storage time for products on hold, and less waste of truly safe food. Reduce potential errors to ensure tests are run properly using the BAX® System’s intuitive layout of software interface and internal positive control tablets.
| Catalog No. | Description | Quantity | |
| KIT2018 | BAX® System Real-time Assay for Campylobacter | 96 Tests per kit |
BAX® System PCR Assays for Cronobacter

BAX® System PCR Assays for Cronobacter
Detect Cronobacter (aka E. sakazakii) with the BAX System PCR Assay for Cronobacter to harness the power of polymerase chain reaction (PCR). This bacteria thrives in matrices and production environments for infant formula and other dried soy and dairy products. Customers worldwide have adopted the BAX® system for the dramatic impact of reducing false-positive results, minimizing the need to re-test, reducing employee training and accelerating the time to market, to ensure safe and wholesome products are provided to consumers.
Catalog Number | KIT2001
Targets | Cronobacter spp (formerly known as E. sakazakii)
PCR Time | 3.5 hours
Certificates | Health Canada
- Targets: Cronobacter spp (formerly known as E. sakazakii)
- Matrices: Powdered infant formula, dry dairy and soy ingredients, environmentals
- Enrichment time: 23-25 hours, depending on food type, sample volume and enrichment media
- PCR time: 3.5 hours
- Detection level: 105 CFU
Increased
Lab Productivity

Simplified
Workflow

Accurate
Results

24-Hour
Support

Dedicated
Applications Lab

BAX® System PCR Tablets
These convenient BAX® System PCR tablets contain all the reagents needed for fast and reliable testing results. Simply hydrate with the prepared sample and load the rack into the BAX® System instrument for automated analysis.
Most Validated Matrices
With decades of experience in food safety, working with hundreds of top food manufacturers across a broad array of product groups, we’ve established dozens of protocols for the most difficult matrices. Our world-class applications scientists have solved the problem, so you don’t have to.
Accurate Results
In the food & beverage industry, accurate results mean fewer re-tests, shorter storage time for products on hold, and less waste of truly safe food. Reduce potential errors to ensure tests are run properly using the BAX® System’s intuitive layout of software interface and internal positive control tablets.
| Catalog No. | Description | Quantity | |
| KIT2001 | BAX® System Assay for Cronobacter | 96 tests per kit |
BAX® System PCR Assays for E. coli O157:H7

BAX® System PCR Assays for E. coli O157:H7
The BAX® System portfolio of E. coli assays offers consistent accuracy, reliability and convenience with flexibility to meet the diverse testing needs of food manufacturers, service labs and government inspection labs. Compare the features among BAX® System E. coli assays in the chart below.
BAX® System PCR Tests for E. coli O157:H7

Real-Time E.coli O157:H7 EXACT
Catalog Number | KIT2039
Targets | E. coli O157:H7
PCR Time | 65 min
Certificates | AOAC-RI PTM

Real-Time E. coli O157:H7
Catalog Number | KIT2000
Targets | E. coli O157:H7
PCR Time | 65 min
Certificates | AOAC-RI PTM, AFNOR, Health Canada, and USDA FSIS

E. coli O157:H7 MP
Catalog Number | KIT2004
Targets | E. coli O157:H7
PCR Time | 3 hrs, 30 min
Certificates | AOAC-RI PTM, AFNOR, and Health Canada

E. coli O157:H7 for X5
Catalog Number | KIT2022
Targets | E. coli O157:H7
PCR Time | 3 hrs, 30 min
Certificates | AOAC-RI PTM and AFNOR
| Assay Description | Targets | PCR Time | Certificates |
| Real-Time E.coli O157:H7 EXACT | E. coli O157:H7 | 65 min | AOAC-RI PTM |
| Real-Time E. coli O157:H7 | E. coli O157:H7 | 65 min | AOAC-RI PTM, AFNOR, Health Canada, and USDA FSIS |
| E. coli O157:H7 MP | E. coli O157:H7 | 3 hrs, 30 min | AOAC-RI PTM, AFNOR, and Health Canada |
| E. coli O157:H7 X5 | E. coli O157:H7 | 8-24 hrs | AOAC-RI PTM and AFNOR |
Increased
Lab Productivity

Simplified
Workflow

Accurate
Results

24-Hour
Support

Dedicated
Applications Lab

BAX® System PCR Tablets
These convenient BAX® System PCR tablets contain all the reagents needed for fast and reliable testing results. Simply hydrate with the prepared sample and load the rack into the BAX® System instrument for automated analysis.
Most Validated Matrices
With decades of experience in food safety, working with hundreds of top food manufacturers across a broad array of product groups, we’ve established dozens of protocols for the most difficult matrices. Our world-class applications scientists have solved the problem, so you don’t have to.
Accurate Results
In the food & beverage industry, accurate results mean fewer re-tests, shorter storage time for products on hold, and less waste of truly safe food. Reduce potential errors to ensure tests are run properly using the BAX® System’s intuitive layout of software interface and internal positive control tablets.
| Catalog No. | Description | Quantity | |
| KIT2039 | BAX® System Q7 Real-ti me Assay E. coli O157:H7 EXACT | 96 tests per kit | |
| KIT2000 | BAX® System Q7 Real-time Assay E. coli O157:H7 | 96 tests per kit | |
| KIT2004 | BAX® System Q7 Real-time Assay E. coli O157:H7 MP | 96 tests per kit | |
| KIT2022 | BAX® System X5 Real-time Assay E. coli O157:H7 | 64 tests per kit |
BAX® System PCR Assays for Genus Listeria

BAX® System PCR Assays for Genus Listeria
Utilization of a Real-Time PCR Genus Listeria assay can reduce total time to product release and corrective action response. With a shortened, simplified sample preparation procedure and rapid real-time processing, this BAX® System assay provides a fast and accurate molecular testing method for Listeria species in food and environmental samples.
| Assay Description | Targets | Enrichment Time | PCR Time | Detection Level | Certificates |
| Real-time Genus Listeria | Genus Listeria | 24-28 hours* | 1.2 hours | 104 CFU | AOAC-RI , AFNOR, Health Canada |
| Genus Listeria 24E | Listeria spp. (except L. grayii) | 24-28 hours | 3.5 hours | 104 CFU | AOAC PTM, AFNOR, and Health Canada |
| Genus Listeria | Listeria spp. | ~48 hours** | 3.5hours | 105 CFU | AOAC PTM, Health Canada |
| Genus Listeria for BAX X5 System | Listeria spp. | ~48 hours* | 3.5 hours | 105 CFU | AOAC PTM, AFNOR, and Health Canada |
*In 24 LEB Complete or 44-72 hours in standard media.
**Enrichment time depends on food type, sample volume and enrichment media
Increased
Lab Productivity

Simplified
Workflow

Accurate
Results

24-Hour
Support

Dedicated
Applications Lab

BAX® System PCR Tablets
These convenient BAX® System PCR tablets contain all the reagents needed for fast and reliable testing results. Simply hydrate with the prepared sample and load the rack into the BAX® System instrument for automated analysis.
Most Validated Matrices
With decades of experience in food safety, working with hundreds of top food manufacturers across a broad array of product groups, we’ve established dozens of protocols for the most difficult matrices. Our world-class applications scientists have solved the problem, so you don’t have to.
Accurate Results
In the food & beverage industry, accurate results mean fewer re-tests, shorter storage time for products on hold, and less waste of truly safe food. Reduce potential errors to ensure tests are run properly using the BAX® System’s intuitive layout of software interface and internal positive control tablets.
| Catalog No. | Description | Quantity | ||
| KIT2019 | D15131113 | BAX® System Real-Time Genus Listeria Assay | 96 tests per kit | |
| KIT2003 | BAX® System Genus Listeria 24E | 96 tests per kit | ||
| KIT2016 | BAX® System Genus Listeria | 96 tests per kit | ||
| KIT2024 | BAX® System X5 PCR Assay for Genus Listeria | 64 tests per kit |
BAX® System PCR Assays for Listeria monocytogenes

BAX® System PCR Assays for Listeria monocytogenes
The BAX® System portfolio of L. mono assays have been designed to meet your needs, whether you’re testing food or environmental samples.
| Assay Description | Targets | Enrichment Time | PCR Time | Detection Level | Certificates |
| Real-time L. monocytogenes | Listeria monocytogenes | 24-28 hours* | 1.2 hours | 104 CFU | AOAC-RI PTM, AOAC-OMA, USDA FSIS, and Health Canada |
| L. monocytogenes 24E | L. monocytogenes | 24-28 hours** | 3.5 hours | 104 CFU | AOAC-RI PTM, AFNOR |
| L. monocytogenes | Listeria monocytogenes | ~48 hours | 3.5 hours | 104 CFU | AOAC-RI PTM, AOAC-OMA, USDA FSIS, and Health Canada |
| L. monocytogenes for BAX® System X5 | Listeria monocytogenes | ~48 hours | 3.5 hours | 105 CFU | AOAC-RI PTM, AFNOR, and Health Canada |
*In 24 LEB Complete or 44-72 hours in standard media.
**Enrichment time depends on food type, sample volume and enrichment media
Increased
Lab Productivity

Simplified
Workflow

Accurate
Results

24-Hour
Support

Dedicated
Applications Lab

BAX® System PCR Tablets
These convenient BAX® System PCR tablets contain all the reagents needed for fast and reliable testing results. Simply hydrate with the prepared sample and load the rack into the BAX® System instrument for automated analysis.
Most Validated Matrices
With decades of experience in food safety, working with hundreds of top food manufacturers across a broad array of product groups, we’ve established dozens of protocols for the most difficult matrices. Our world-class applications scientists have solved the problem, so you don’t have to.
Accurate Results
In the food & beverage industry, accurate results mean fewer re-tests, shorter storage time for products on hold, and less waste of truly safe food. Reduce potential errors to ensure tests are run properly using the BAX® System’s intuitive layout of software interface and internal positive control tablets.
| Catalog No. | Description | Quantity | |
| KIT2005 | BAX® System Real-Time L. monocytogenes | 96 test per kit | |
| KIT2002 | BAX® System L. monocytogenes 24E | 96 test per kit | |
| KIT2017 | BAX® System L. monocytogenes | 96 test per kit | |
| KIT2023 | BAX® System X5 PCR Assay for L. monocytogenes | 96 test per kit |
BAX® System PCR Assays for Salmonella

BAX® System PCR Assays for Salmonella
Food processors and associated laboratories around the world use the BAX® System for accurate, reliable Salmonella detection in raw ingredients, finished products and environmental samples. Now, the BAX® System Real-time PCR Assay for Salmonella provides the same clear, dependable results even faster.
| Assay Description | Targets | PCR Time | Certificates |
| Real-time Salmonella | Salmonella spp. | 75 min | AOAC-OMA, AOAC-RI PTM, AFNOR |
| Salmonella | Salmonella sp. | 3 hrs, 30 min | AOAC-OMA, AOAC-RI PTM, AFNOR, NordVal, and Health Canada |
| Salmonella for BAX® System X5 | Salmonella sp. | 3 hrs, 30 min | AOAC-RI PTM and AFNOR |
Increased
Lab Productivity

Simplified
Workflow

Real-Time
Quantification

24-Hour
Support

Dedicated
Applications Lab

BAX® System PCR Tablets
These convenient BAX® System PCR tablets contain all the reagents needed for fast and reliable testing results. Simply hydrate with the prepared sample and load the rack into the BAX® System instrument for automated analysis.
Most Validated Matrices
With decades of experience in food safety, working with hundreds of top food manufacturers across a broad array of product groups, we’ve established dozens of protocols for the most difficult matrices. Our world-class applications scientists have solved the problem, so you don’t have to.
Accurate Results
In the food & beverage industry, accurate results mean fewer re-tests, shorter storage time for products on hold, and less waste of truly safe food. Reduce potential errors to ensure tests are run properly using the BAX® System’s intuitive layout of software interface and internal positive control tablets.
| Catalog No. | Description | Quantity | |
| KIT2006 | BAX® System Real-Time Salmonella Assay | 96 tests per kit | |
| KIT2012 | BAX® System Salmonella Assay | 96 tests per kit | |
| KIT2025 | BAX® System Salmonella for X5 Assay | 32 per run, 64 per kit |
BAX® System PCR Assays for Shigella

BAX® System PCR Assays for Shigella
Shigella naturally shares large portions of its’ genetic sequence with many other pathogenic bacteria and is very easy to cross-contaminate with very low dose response required. This cross-contamination primarily occurs from poor hygiene practices during food production and handling in retail environments. Utilizing the Real-time PCR assay for Shigella can quickly eliminate negative samples and reduce presumptive positives seen in conventional methods due to the specificity and inclusivity of serotypes. Common implicated serotypes including S. sonnei, S. flexneri, S. boydii, and S. dysenteriae are easily detected to help you identify potential contamination quickly.
Catalog Number | KIT2007
Targets | Shigella spp.
PCR Time | 65 min
Certificates | Internally validated
- Catalog Number: KIT2007
- Targets: Shigella spp.
- PCR Time: 65 min
- Certificates: Internally validated
Increased
Lab Productivity

Simplified
Workflow

Accurate
Results

24-Hour
Support

Dedicated
Applications Lab

BAX® System PCR Tablets
These convenient BAX® System PCR tablets contain all the reagents needed for fast and reliable testing results. Simply hydrate with the prepared sample and load the rack into the BAX® System instrument for automated analysis.
Most Validated Matrices
With decades of experience in food safety, working with hundreds of top food manufacturers across a broad array of product groups, we’ve established dozens of protocols for the most difficult matrices. Our world-class applications scientists have solved the problem, so you don’t have to.
Accurate Results
In the food & beverage industry, accurate results mean fewer re-tests, shorter storage time for products on hold, and less waste of truly safe food. Reduce potential errors to ensure tests are run properly using the BAX® System’s intuitive layout of software interface and internal positive control tablets.
| Catalog No. | Description | Quantity | |
| KIT2007 | BAX® System Real-Time Assay for Shigella | 96 tests per kit |
BAX® System PCR Assays for Staphylococcus aureus

BAX® System PCR Assays for Staphylococcus aureus
Staphylococcus aureus is a common cause of skin infections, and is known to cause foodborne illness and methycillin-resistant Staph aureus is a significant problem in clinical settings. The BAX® System Real-Time PCR assay for Staphylococcus aureus harnesses the power of the polymerase chain reaction to detect Staph aureus in food and environmental samples. The BAX® System provides everything necessary for PCR–polymerase, nucleotides and target-specific primers. The system minimizes operator handling and is easy to use. It can be used for presence/absence or threshold testing.
Catalog Number | KIT2020
Targets | Staphylococcus aureus
PCR Time | 70 min
Certificates | AOAC-RI PTM
- Catalog Number: KIT2020
- Targets: Staphylococcus aureus
- PCR Time: 70 min
- Certificates: AOAC-RI PTM
Increased
Lab Productivity

Simplified
Workflow

Accurate
Results

24-Hour
Support

Dedicated
Applications Lab

BAX® System PCR Tablets
These convenient BAX® System PCR tablets contain all the reagents needed for fast and reliable testing results. Simply hydrate with the prepared sample and load the rack into the BAX® System instrument for automated analysis.
Most Validated Matrices
With decades of experience in food safety, working with hundreds of top food manufacturers across a broad array of product groups, we’ve established dozens of protocols for the most difficult matrices. Our world-class applications scientists have solved the problem, so you don’t have to.
Accurate Results
In the food & beverage industry, accurate results mean fewer re-tests, shorter storage time for products on hold, and less waste of truly safe food. Reduce potential errors to ensure tests are run properly using the BAX® System’s intuitive layout of software interface and internal positive control tablets.
| Catalog No. | Description | Quantity | |
| KIT 2020 | BAX® System Real-Time Assay for Staph aureus | 96 tests per kit |
BAX® System PCR STEC Screening Suite

BAX® System PCR STEC Screening Suite
The BAX® System portfolio of Real-time STEC Screening assays is designed to identify the top six non-O157 Shiga toxin-producing E. coli (STEC) defined by USDA Food Safety and Inspection Service (FSIS) as adulterants in the beef industry. Compare the features among BAX® System STEC Screening assays in the chart below.
BAX® System Real-Time PCR Assays for STEC Screening
Three STEC screening test kits and dozens of validated matrices.

Real-Time STEC Screening
Catalog Number | KIT2021
Targets | stx and eae genes – STEC Screening
PCR Time | 1.5 hours
Certificates | AOAC-RI PTM

Real-Time STEC Panel 1
Catalog Number | KIT2008
Targets | E. coli O26, O111, O121
PCR Time | 1.5 hours
Certificates | AOAC-RI PTM

Real-Time STEC Panel 2
Catalog Number | KIT2009
Targets | E. coli O45, O103, O145
PCR Time | 1.5 hours
Certificates | AOAC-RI PTM
| Assay Description | Targets | Enrichment Time | PCR Time | Detection Level | Certificates |
| Real-Time STEC Screening | stx and eae genes – STEC Screening | 10-24 hours* | 90 minutes | 104 CFU | AOAC PTM |
| Real-Time STEC Panel 1 | E. coli O26, O111, O121 | 10-24 hours* | 90 minutes | 104 CFU | AOAC PTM |
| Real-Time STEC Panel 2 | E. coli O45, O103, O145 | 10-24 hours* | 90 minutes | 104 CFU | AOAC PTM |
| Salmonella for BAX® System X5 | Listeria monocytogenes | 10-24 hours* | 3.5 hours | 104 CFU | AOAC PTM |
*Enrichment time depends on food type, sample volumne and enrichment media
Increased
Lab Productivity

Simplified
Workflow

Accurate
Results

24-Hour
Support

Dedicated
Applications Lab

BAX® System PCR Tablets
These convenient BAX® System PCR tablets contain all the reagents needed for fast and reliable testing results. Simply hydrate with the prepared sample and load the rack into the BAX® System instrument for automated analysis.
Most Validated Matrices
With decades of experience in food safety, working with hundreds of top food manufacturers across a broad array of product groups, we’ve established dozens of protocols for the most difficult matrices. Our world-class applications scientists have solved the problem, so you don’t have to.
Accurate Results
In the food & beverage industry, accurate results mean fewer re-tests, shorter storage time for products on hold, and less waste of truly safe food. Reduce potential errors to ensure tests are run properly using the BAX® System’s intuitive layout of software interface and internal positive control tablets.
| Catalog No. | Description | Quantity | |
| KIT2021 | BAX® System Real-Time PCR Assay for STEC Screening | 96 tests per kit | |
| KIT2008 | BAX® System Real-Time PCR Assay for STEC Panel 1 | 48 tests per kit | |
| KIT2009 | BAX® System Real-Time PCR Assay for STEC Panel 2 | 48 tests per kit |
BAX® System Real-Time PCR Assays for Vibrio

BAX® System Real-Time PCR Assays for Vibrio
Vibrio is a persistent threat to producers (and consumers) of seafood, and is easily spread through coastal waters and brackish aquatic environments. Current culture methods are accurate but can take 3 to 5 days to get results. The BAX System Real-Time PCR Assay for Vibrio detects (V. cholerae, V. parahaemolyticus, V. vulnificus) and harnesses the power of the polymerase chain reaction (PCR) to get results in 24 hours, so you can detect and differentiate three distinct pathogenic species in one test, so you can take action without delaying product release to commerce.
Catalog Number | KIT2010
Targets | V. cholerae, V. parahaemolyticus, V. vulnificus
PCR Time | 70 min
Certificates | AOAC-RI PTM
- Catalog Number: KIT2010
- Targets: V. cholerae, V. parahaemolyticus, V. vulnificus
- PCR Time: 70 min
- Certificates: AOAC PTM
Increased
Lab Productivity

Simplified
Workflow

Accurate
Results

24-Hour
Support

Dedicated
Applications Lab

BAX® System PCR Tablets
These convenient BAX® System PCR tablets contain all the reagents needed for fast and reliable testing results. Simply hydrate with the prepared sample and load the rack into the BAX® System instrument for automated analysis.
Most Validated Matrices
With decades of experience in food safety, working with hundreds of top food manufacturers across a broad array of product groups, we’ve established dozens of protocols for the most difficult matrices. Our world-class applications scientists have solved the problem, so you don’t have to.
Accurate Results
In the food & beverage industry, accurate results mean fewer re-tests, shorter storage time for products on hold, and less waste of truly safe food. Reduce potential errors to ensure tests are run properly using the BAX® System’s intuitive layout of software interface and internal positive control tablets.
| Catalog No. | Description | Quantity | |
| KIT2010 | BAX® System Real-Time PCR Assay for Vibrio | 96 tests per kit |
BAX® System PCR Assays for Yeast & Mold

BAX® System PCR Assays for Yeast & Mold
Yeast and mold cause various degrees of deterioration and decomposition of many food products. Although these bacteria do not cause many human illnesses, they are spore formers that can release toxins once ingested. Moreover, yeast and molds can negatively impact the shelf life and appeal of any product, thus causing economic losses. Compared to conventional methods that take up to 7 days, the BAX® System PCR assay for Yeast and Mold provides results in two days for enriched products, and same-day results when product is direct tested. The protocols associated with this assay are customizable for actionable thresholds, proving the true power and robustness of PCR technology.
Catalog Number | KIT2015
Targets | Yeast and Mold
PCR Time | 3 hrs 30 min
Certificates | AOAC-RI PTM
- Catalog Number: KIT2015
- Target: Yeast and mold
- PCR time: 3 hrs
- Certification: AOAC-RI PTM
Increased
Lab Productivity

Simplified
Workflow

Accurate
Results

24-Hour
Support

Dedicated
Applications Lab

BAX® System PCR Tablets
These convenient BAX® System PCR tablets contain all the reagents needed for fast and reliable testing results. Simply hydrate with the prepared sample and load the rack into the BAX® System instrument for automated analysis.
Most Validated Matrices
With decades of experience in food safety, working with hundreds of top food manufacturers across a broad array of product groups, we’ve established dozens of protocols for the most difficult matrices. Our world-class applications scientists have solved the problem, so you don’t have to.
Accurate Results
In the food & beverage industry, accurate results mean fewer re-tests, shorter storage time for products on hold, and less waste of truly safe food. Reduce potential errors to ensure tests are run properly using the BAX® System’s intuitive layout of software interface and internal positive control tablets.
Instruments
BAX System Q7
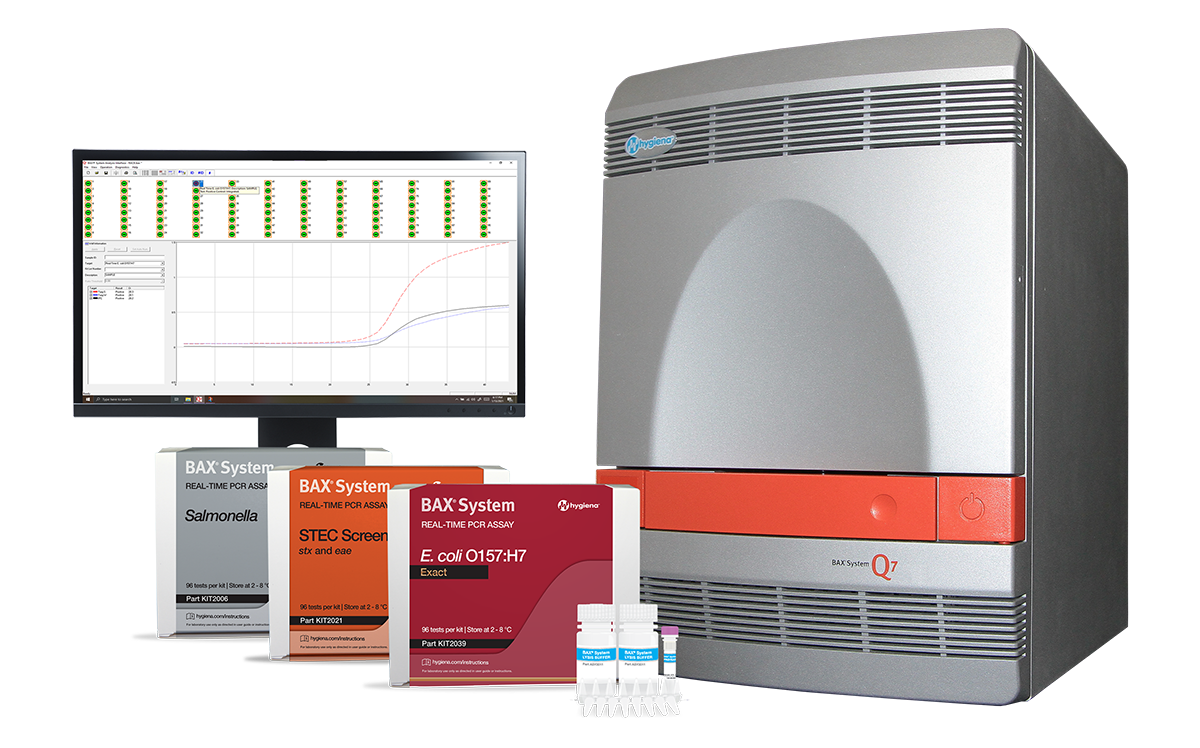
BAX® System Q7
Powerful and Precise Molecular Pathogen Detection
The original creators of PCR technology currently provide over two decades of reliable food safety testing with the largest breadth of validated matrices. The BAX® System continues to evolve to support food safety needs through simplified testing methods, specific and sensitive assays, and real-time PCR quantification.
Instrument Specifications
- Dimensions: 14″ wide x 18″ deep x 20″ high (34 x 45 x49 cm)
- Weight: Approx. 75 pounds (34.1 kg)
- Power usage: 950 watts
- Nominal current draw: 8 A
- Power requirements: 100-240 VAC ±10%, 50-60 Hx ± 1%, 15 amp circuit, grounded outlet
- Fuses: 5 excitation, 5 emission
- Halogen bulb: 12 V, 75 W
- Thermal range: 4-100ºC
- Thermal homogeneity: ±1.0º well-to-well within 30 seconds of reaching 60ºC
- Thermal accuracy: ±0.5ºC (35ºC-95ºC) 3 minutes after reaching set point temperature
- Ramp speed: ±1.6-3.5ºC per second
- Warm up time: 5 minutes or less from 25º C start
- Sound: 65 decibels maximum
- Sample capacity: 1-96 test per batch, time dependent on assay
Environmental Parameters
- Altitude at or below 2000 m (6500 ft) above sea level
- Temperature 10-35ºC (50-95ºF)
- Relative humidity 20-80% (non-condensing)
- Locate away from heaters, cooling ducts, and out of direct sunlight
- Protect from accidental spills
- Non-conductive pollutants only
Delivered System
- Computer workstation with CD read/write drive, monitor, keyboard, mouse and cables
- Microsoft® Windows® operating system, BAX® System application and BAX® System documentation package
Increased
Lab Productivity

Simplified
Workflow

Accurate
Results

24-Hour
Support

Dedicated
Applications Lab

BAX® System PCR Tablets
These convenient BAX® System PCR tablets contain all the reagents needed for fast and reliable testing results. Simply hydrate with the prepared sample and load the rack into the BAX® System instrument for automated analysis.
Most Validated Matrices
With decades of experience in food safety, working with hundreds of top food manufacturers across a broad array of product groups, we’ve established dozens of protocols for the most difficult matrices. Our world-class applications scientists have solved the problem, so you don’t have to.
Accurate Results
In the food & beverage industry, accurate results mean fewer re-tests, shorter storage time for products on hold, and less waste of truly safe food. Reduce potential errors to ensure tests are run properly using the BAX® System’s intuitive layout of software interface and internal positive control tablets.
| Catalog No. | Description | Quantity | ||
| ASY2018 | BAX® System Screener EQ Pkg (120V) | 1 | ||
| ASY2020 | BAX® System Screener EQ Pkg (220V) | 1 | ||
| ASY2016 | BAX® System Screener EQ Pkg with Digital Heating Block | 1 | ||
| ASY2013 | BAX® System Q7 Workstation | 1 | ||
| ASY2014 | Auxiliary Package for BAX® System Workstation (120V) | 1 | ||
| ASY2015 | Auxiliary Package for BAX® System Workstation (220V) | 1 | ||
| MCH2019 | BAX® System Q7 Windows Upgrade Package with or without ESA | 1 | ||
| MCH2014 | D11676874 | Heating Block (120V) Double Block System | 1 | |
| MCH2026 | Heating Block (220V) Double Block System | 1 | ||
| MIS2021 | Dry Block Insert | 1 | ||
| MIS2030 | Insulator For Both Lysate & PCR Blocks | |||
| MIS2017 | Cooling Blocks | |||
| MIS2039 | PCR Cooling Block (Insert Only) | |||
| MCH2023 | Thermal Block | |||
| MCH2011 | Cell Disrupter for Yeast & Mold (120V) | |||
| MCH2012 | Cell Disrupter for Yeast & Mold (220V) | |||
| KIT2026 | Calibration Kit |
BAX System X5


Accurate and Reliable Results
Advanced DNA-based PCR technology provides rapid, reproducible results to help you make faster product release decisions with confidence.
The BAX® System X5 handles up to 32 samples using the same advanced PCR technology to accurately and reliably identify foodborne pathogens in raw ingredients, finished products, and environmental samples with a smaller lab footprint.
Instrument Specifications
- Dimensions: 9.45 in x 10.63 in x 9.06 in (24 cm x 27 cm x 23 cm)
- Weight: Approx. 15.4 lbs (7 kg)
- Power supply: 100-240 VAC; 50-60 Hz
- Power usage: Operates at 24 V with 6.25 A; maximum usage 170 VA
- Mains power consumption: 170 W
- Noise level: < 40 dB (A)
- Heat output during run (mean value): ~58 Btu/h or 170 kJ/h
- Heat output in standby: ~34 Btu/h or 36 kJ/h
- Sample capacity: Up to 32 samples per run
- Processing time: 3.5 hours for standard PCR protocols
- Fluorescence spectrometer excitation: Filtered LED, 500 nm
- Fluorescence spectrometer detection: Spectrometer, 2 nm (emission wavelength)
- Channels: 120 optical channels
- Temperature range: 40-99 °C
- Heating rate: 4 °C/s
- Cooling rate: 3 °C/s
- Thermal homogeneity: ±0.25 °C
- Thermal accuracy: ±0.50 °C
Environmental Parameters
- Minimum bench space required (width x depth x height): 9.45 in x 12.21 in x 14.96 in (24 cm x 32 cm x 38 cm)
- Temperatures allowed during transportation, storage and packaging: -20 to +60 °C
- Relative humidity allowed during transportation, storage and packaging: 10-95%, no condensation
- Altitude/pressure allowed during transportation, storage and packaging: 0-3,000 m above sea level / 70-106 kPa
- Temperatures allowed during operation: 15-32 °C
- Relative humidity allowed during operation: Max. 80% at 32 °C, no condensation / Min. 30% at 15-32 °C
- Alititude/pressure allowed during operation: 0-2,000 m above sea level / 80-106 kPa
Delivered System
- Computer workstation with CD read/write drive, monitor, keyboard, mouse and cables
- Microsoft® Windows® operating system, BAX® System application and BAX® System documentation package
Increased
Lab Productivity

Simplified
Workflow

24-Hour
Support

Dedicated
Applications Lab

BAX® System PCR Tablets
These convenient BAX® System PCR tablets contain all the reagents needed for fast and reliable testing results. Simply hydrate with the prepared sample and load the rack into the BAX® System instrument for automated analysis.
Most Validated Matrices
With decades of experience in food safety, working with hundreds of top food manufacturers across a broad array of product groups, we’ve established dozens of protocols for the most difficult matrices. Our world-class applications scientists have solved the problem, so you don’t have to.
Accurate Results
In the food & beverage industry, accurate results mean fewer re-tests, shorter storage time for products on hold, and less waste of truly safe food. Reduce potential errors to ensure tests are run properly using the BAX® System’s intuitive layout of software interface and internal positive control tablets.
| Catalog No. | Description | Quantity | |
| MCH2007 | BAX® System X5 Startup Package | 1 | |
| MCH2005 | BAX® System X5 Instrument Package | 1 | |
| MCH2003 | BAX® System X5 Computer Package | 1 | |
| MCH2000 | BAX® System X5 Auxiliary Package | 1 | |
| MCH2008 | BAX® System X5 32-well Sample Holder | 1 | |
| MCH2001 | BAX® System X5 32-well Aluminum Cold Block | 1 | |
| MCH2002 | BAX® System X5 Insulator for PCR Cold Block | 1 | |
| ASY2065 | BAX® System X5 Empty PCR Tubes | 12 strips of 8 | |
| KIT2022 | BAX® System X5 PCR Assay for E. coli O157:H7 | 32 per run, 64 per kit | |
| KIT2024 | BAX® System X5 PCR Assay for Genus Listeria | 32 per run, 64 per kit | |
| KIT2023 | BAX® System X5 PCR Assay for L. monocytogenes | 32 per run, 64 per kit |
BAX Prep Xpress

BAX Prep Xpress
The Right Fit for Any Lab
Hygiena’s BAX® Prep Xpress is an affordable, small footprint, automated liquid handling platform that reduces technician labor while increasing pipetting accuracy and reliability. The high throughput workflow empowers autonomy for other laboratory tasks while improving efficiency and consistency of routine solution preparation.
Power
- Input Power (Primary), Universal Supply: 100-240 VAC, 50-60 Hz
- Output Power (Secondary): 500 W at 48 V
Physical Dimensions
- Width: 21 in / 534 mm
- Depth (with screen): 25 in / 635 mm
- Height: 24 in / 610 mm
- Height (door open): 32 in / 813 mm
- Weight : 91.5 lbs / 41.6 kg
Operation
- Deck Capacity: Up to 8 positions
- Plate Transport Mass: Maximum 300 g filled deep well plate
- Communication: USB, Ethernet
- Operating Temperature: 15-35 °C (59–95 °F)
- Relative Humidity: 15-85% non-condensing
Storage
- Storage Temperature: -20-70 °C
- Relative Humidity: 10-90% non-condensing
Increased
Lab Productivity

Simplified
Workflow

Accurate
Results

24-Hour
Support

Dedicated
Applications Lab

Increase Lab Productivity
Demonstrated reduction in solution preparations resulting in the more productive use of lab technician time.
Small Footprint
Compact 2 ft3 size eliminates the need for extra benchtop space that can be utilized for other routine testing needs.
Streamlined Workflow
Continuous workflow eliminates interruption from the sampling process to maximize efficiencies, not only in PCR prep but in all areas of lab work.
On-Board Control
Minimizes downtime with on-board tracking software that prompts technicians for maintenance activities and eliminates the need for paperwork.
RiboPrinter System

RiboPrinter System
Patented Process. Automated Performance.
Only the RiboPrinter® System from Hygiena™ combines automation with the power of DNA to easily and accurately provide microbial identification & characterization of contaminants, as well as track their source at the strain level–helping you gain control of any microbial environment.
- Processes up to 8 concurrent samples
- Onboard ID library
- Over 8,500 patterns
- 296 bacterial genre
- 1,740 species and serotypes
Increased
Lab Productivity

Simplified
Workflow

Accurate
Results

24-Hour
Support

Dedicated
Applications Lab

With the Powerful RiboPrinter System:
- Patterns can be used to identify genus and species.
- Loading and operating the characterization unit are easy and intuitive.
- Automated RiboPrinter® System simplifies operator training and minimizes errors due to technique.
- Workstation software is user-friendly, providing sophisticated data analysis that eliminates the need for subjective interpretation of results.

Gain Control of Your Environment
RiboPrint™ patterns characterize environmental isolates, pathogens, spoilage organisms, control strains, beneficial organisms or any bacteria that are important to quality control professionals in pharmaceuticals, food & beverage, and industrial microbiology. Beyond reliable taxonomic identification, the RiboPrinter® System distinguishes individual strains, providing the granularity of information needed to track, trend, and control your environment.
Identify, Characterize, and Trace
To control unwanted bacteria, you need to know what the organism is and how it entered your process or population. Now you can get the answers to these important questions automatically and simultaneously.


More Than 16S Sequencing
Rather than just analyzing the region that encodes the 16s rRNA sequence, the RiboPrinter® System investigates the regions encoding the 5S, 16S, and 23S sequences, as well as the spacer regions and flanking genes on either side. This rich depth of information is what allows for highly precise differentiation among strains of the same species, even those with the same 16S sequence.


Fragments from each sample form a banding pattern according to size.

Banding patterns & marker lanes captured in a digitized gel image.
Final report displays the RiboPrint™ pattern and associated
data for each sample.
| Catalog No. | Description | Quantity | Contact Sales |
| Instruments | |||
| ASY2005 | RiboPrinter® System Instrument – Windows® OS 220V | 1 | |
| ASY2004 | RiboPrinter® System Instrument – Windows® OS 110V | 1 | |
| N/A | RiboPrinter® System 2.3 Software | 1 | |
| ASY2074 | RiboPrinter® System Windows® 7 Upgrade Package 110V w/ or w/o ESA | 1 | |
| Reagents | |||
| ASY2077 | RiboPrinter® System Disposable Assembly Kit EcoR I (48 tests) Includes Purified Water | 1 | |
| MCH2050 | RiboPrinter® System Disposable Assembly Kit EcoR I (48 tests) Without Purified Water | 48 tests | |
| ASY2078 | RiboPrinter® System Disposable Assembly Kit Pvu II (48 tests) Includes Purified Water | 48 tests | |
| ASY2072 | RiboPrinter® System Disposable Assembly Kit Pvu II Without (48 tests) Purified Water | 48 tests | |
| ASY2071 | RiboPrinter® System Disposable Assembly Kit – No Enzyme (48 tests) Includes purified water | 48 tests | |
| ASY2076 | RiboPrinter® System Disposable Assembly Kit – No Enzyme (48 tests) Without purified water | 48 tests | |
| KIT2032 | RiboPrinter® System DNA Prep-PVU II Frozen Enzyme Kit | 1 | |
| KIT2029 | RiboPrinter® System DNA Prep-Eco RI Frozen Enzyme Kit | 1 | |
| KIT2031 | RiboPrinter® System DNA Prep-No Enzyme Kit (FR) | 1 | |
| KIT2034 | RiboPrinter® System/MP Base Pack | 1 | |
| KIT2036 | RiboPrinter® System/System Water | ||
| KIT2033 | RiboPrinter® System-Gel Casette & Memb. Box | ||
| KIT2030 | RiboPrinter® System-MP Inserts & DNA Prep Kits | ||
| KIT2035 | Sample Preparation Pack Set Box |
Automated Thermo Blocks

Automated Thermo Blocks
Automate Lysis Step Heating and Cooling
The Hygiena™ Automated Thermal Block is an automated heating and cooling device that is programmed to perform the lysis steps of BAX® System protocols. By automatically providing sequential heating and cooling conditions, the Hygiena™ Thermal Block eliminates the need to manually transfer samples between separate heating and cooling blocks. At the end of each lysis program, the block holds the sample at 4 °C until you remove them.
- Heating & Cooling Blocks
- AOAC-RI approved for use with most BAX® System assays
Increased
Lab Productivity

Simplified
Workflow

24-Hour
Support

How to Use
| Catalog No. | Description | Quantity | |
| DMCH2023 | Hygiena™ Automated Thermal Block | 1 ea |
Quantification
BAX System SalQuant

BAX System SalQuant
First Real-Time PCR Application of Quantification
Hygiena™ is pioneering True Quantification of Salmonella as a unified resource across the full production chain. BAX® SalQuant™ is an easy-to-use, affordable solution providing rapid, actionable decisions for the poultry, beef, and pork industries.
What happens when a sample tests positive? How actionable is presence/absence? What if you could know a load of Salmonella in a positive sample within the same shift or day?
- 96-well format for maximum sample throughput
- Robust validations including AOAC and AFNOR
- Clear and reproducible results, independent of operator technique
- Internal positive control with every assay to validate negative results
- Continuous detection during PCR cycling collects data in “real-time” reducing time to result
- Automated cycling, detection and analysis without the need for expert skill
- Multi-wavelength real-time detection to identify multiple targets in a single sample
- LIMS compatible system allows for easy storage, retrieval and printing
BAX® System SalQuant™ Tested Matrices
Over 85,000 samples ran to date on various matrices to generate the protocols.
Available Protocols for Matrices:
Poultry
- Comminuted Chicken or Turkey – AOAC-RI PTM℠
- Whole Bird (Carcass) Rinsates
- Parts Rinsates
- Boot, Dust, Feet, Cloacal Swabs
- Poultry Pads (Cardboard or Straw)
- Feed
- Ceca
- Crop
- Lungs
Beef
- Ground & Finely Textured Beef
- Beef Trim
- MicroTally™ Manual Sampling Device for Beef Trim
- Lymph Node
Pork
- Ground Pork
- Pork Trim
- MicroTally
- Head Trim Rinses
- Carcass Swab
- Lymph Node
Quantification Data Applications throughout the Full Value Chain

LIVE PRODUCTION
- Vaccine efficacy
- Pre-Harvest antibiotic/probiotic efficiency
- Flock house sanitation/cleaning monitoring
- Determination of slaughter order
- Source tracking of high load animals for corrective actions at farm level
- Monitoring animal status overtime
- Screening for presence/absence of Salmonella

PROCESSING
- New and existing chemical and physical intervention evaluations
- Biomapping of process locations to monitor intervention efficacy
- Process control documentation support
- Precise knowledge of Salmonella location and level of contamination
- Tracking from incoming lots to final product

FINAL PRODUCT
- Make release decisions faster for ground products contaminated with 1-10 CFU/g or mL (SalLimits™ or SalQuant™)
- Faster results to make diversion decisions
- Determine the true meaning of Salmonella prevalence by applying quantitative values to the results
- Provide support data for prevalence testing to reduce consumer risk when positive lots are released
| Catalog No. | Description | Quantity | |
| KIT2006 | BAX® System Real-Time Salmonella Assay | 96 tests per kit | |
| MED2003 | BAX® System MP Media | 2.5 kg |
Chromogenic
InSite Salmonella

Environmental Salmonella Test
Hygiena™ InSite™ Salmonella is an easy-to-use, self-contained, environmental Salmonella spp. test. Each device contains a liquid medium formulated with growth enhancers and chromogenic compounds selective for Salmonella species. All InSite™ test devices contain neutralizers in the wetting solution to help combat potential sanitizer side effects and improve sample collection. Simply swab the test area and incubate. A change in color after 24-48 hours of incubation is considered presumptive positive for Salmonella species. No expensive lab equipment is required.
The InSite™ Chromo and InSite™ L. mono Glo test devices contain a neutralizing wetting solution on the swab, it is a mixture of Buffered Peptone Water and Neutralizing Broth.
- Snap-Valve technology – Simply snap & squeeze
- Large 2.5 inch foam swab for the maximum sample pickup
- Write-on swab label for easy sample identification
- Presumptive positive results are available in as little as 24 hours
- Presumptive positive samples can be confirmed by streaking samples onto commonly used selective Salmonella agar plates or any recognized confirmatory procedure. Typical Salmonella colonies on selective agar plates could then be further analyzed by using biochemical, immunological, or molecular methods.
- Sensitive to 1-10 CFU
Results in 24-48 Hours

Reduce Material & Labor

Self-Contained Reagent

Easy to Interpret Color Change

Patented Snap-Valve Technology

How to Use

Easy to Interpret Color Results
It doesn’t take a microbiologist to understand these results. When Salmonella species is present in a sample, the liquid medium changes color from purple to vivid yellow.
| Catalog No. | Description | Quantity | |
| IS050 | Insite Salmonella | 50 |
InSite™ L. mono Glo

Environmental L. mono Test
Hygiena™ InSite™ L. mono Glo is an easy-to-use, self-contained, environmental sampling and screening test for Listeria species and Listeria monocytogenes (L. mono). Each device contains a chromogenic liquid media formulated with antibiotics, growth enhancers, and color-changing compounds specific to Listeria plus fluorescent compounds specific to Listeria monocytogenes. Plus, all InSite™ test devices contain neutralizers in the wetting solution to help combat potential sanitizer side effects and improve sample collection. The two-step test changes color in the presence of Listeria species, while illumination with UV light reveals the presence of L.mono.
- Snap-Valve technology – simply snap & squeeze
- Large 2.5 inch foam swab for the maximum sample pickup
- Write-on swab label for easy sample identification
- Presumptive positive results are available for the most common Listeria spp in as little as 24 hours at levels as low as 1-10 CFU/mL
- Presumptive positive samples should be confirmed by an alternative method such as PCR, lateral flow, or traditional bacteriology methods. Any confirmatory results should be handled according to appropriate regulations.
- Compared to UVM and BLEB, provides superior/equivalent recovery and faster detection of as low as 10-50 heat-injured Listeria monocytogenes within ~24-48 hours of incubation
Results in 24-48 Hours

Reduce Material & Labor

Self-Contained Reagent

Easy to Interpret Color Change

Patented Snap-Valve Technology

How to Use

Easy to Interpret Color Results
It doesn’t take a microbiologist to understand these results. When Listeria species are present in a sample, the liquid media changes color from amber to black. If pathogenic Listeria, like L. mono, is present, the media will glow green under UV light.
| Catalog No. | Description | Quantity | |
| ILM050 | InSite™ L. mono GloTest | 50 |
InSite Listeria

Environmental Listeria species Test
Hygiena™ InSite™ Listeria is an easy-to-use, self-contained, environmental Listeria species test. Each device contains a chromogenic liquid media formulated with antibiotics, growth enhancers, and color-changing compounds specific to Listeria species. Plus, all InSite™ test devices contain neutralizers in the wetting solution to help combat potential sanitizer side effects and improve sample collection. Simply swab the test area and wait. A change in color after 24-48 hours of incubation is considered a presumptive positive. No expensive lab equipment is required and no additional sample handling of the sample is necessary.
- Snap-Valve technology – simply snap & squeeze
- Large 2.5 inch foam swab for the maximum sample pickup
- Write-on swab label for easy sample identification
- Presumptive positive results are available for the most common Listeria spp in as little as 30 hours at levels as low as 1-10 CFU/mL
- Presumptive positive samples should be confirmed by streaking samples onto commonly used selective Listeria agar plates such as Modified Oxford agar, Palcam agar, or any other recognized confirmatory procedure. Typical Listeria colonies on selective agar plates could then be further analyzed by more definitive tests such as microscopy, biochemical tests, etc.
- Compared to UVM and BLEB, provides superior/equivalent recovery and faster detection of as low as 10-50 heat injured Listeria monocytogenes/mL within ~24-30 hours of incubation.
- Provides a higher level of selectivity and sensitivity for heat injured L. monocytogenes under binary competition conditions compared to BLEB and UVM media
Results in
24-48 Hours

Reduce
Material & Labor

Self-Contailed
Reagent

Easy to Interpret
Color Change

Patented Snap-Valve Technology

How to Use

Easy to Interpret Color Results
It doesn’t take a microbiologist to understand these results. When Listeria species is present in a sample, the liquid media changes color from amber to black.
| Catalog No. | Description | Quantity | |
| ILC50 | InSite™ Listeria Test (box of 50) | 50 Tests | |
| ILC100 | InSite™ Listeria Test (box of 100) | 100 Tests |
Media
Actero Elite Listeria Enrichment Media

Actero Elite Listeria Enrichment Media
The patented Actero™ Elite Listeria Enrichment Media is a selective bacterial culture medium specifically optimized for the recovery of Listeria spp. and Listeria monocytogenes from environmental and food samples in a single-step enrichment. Actero™ Elite Listeria is an optimized formulation with the advantages of having the fastest time-to-results, and is accurate and specific for the target bacteria even in the presence of abundant competing flora.
- Actero™ Elite Salmonella Enrichment Media
Increased
Lab Productivity

Simplified
Workflow

Accurate
Results

24-Hour
Support

Dedicated
Applications Lab

Maximize Your Competitive Advantage
Faster recovery & detection using BAX® System Real-Time PCR Assay for Listeria with Actero™ Elite Listeria Enrichment Media.
- Single-step enrichment
- As little as 20 hours to results
- Better than or equal to the reference method – Accuracy validated by 3rd party labs (AOAC-RIPTM, Health Canada)

Single-Step Enrichment
Less time spent on prep means cost savings for food manufacturers and third-party reference labs. Actero™ Elite Listeria Enrichment media’s single-step enrichment reduces “time-to-results” and minimizes possible errors, providing faster turnaround for product release decisions.

Robust List of Validated Matrices
These matrices make up some of the most commonly encountered in any food processing environment. Many matrices can interfere with PCR, preventing target amplification. Validated enrichment media can help control this inhibition, ensuring you get the pathogen detection information you need to make key food safety decisions.

| Catalog No. | Description | Quantity | |
| MED2017 | Actero™ Listeria Enrichment Media | 500 g | |
| MED2018 | Actero™ Listeria Enrichment Media | 2 kg | |
| MED2020 | Actero™ Listeria Enrichment Media | 10 kg |
Actero Elite Salmonella Enrichment Media

Actero Elite Salmonella Enrichment Media
The patented Actero™ Elite Salmonella Enrichment Media is a selective bacterial culture medium specifically optimized for the recovery of Salmonella spp. from environmental and food samples in a single-step enrichment. Actero™ Elite Salmonella is an optimized formulation with the advantages of having the fastest time-to-results, and is accurate and specific for the target bacteria even in the presence of abundant competing flora.
- Actero™ Elite Salmonella Enrichment Media – 2 kg
Increased
Lab Productivity

Simplified
Workflow

Accurate
Results

24-Hour
Support

Dedicated
Applications Lab

Maximize Your Competitive Advantage
Faster recovery & detection using BAX® System Real-Time PCR Assay for Salmonella with Actero™ Elite Salmonella Enrichment Media.
- Single-step enrichment
- As short as 16 hours to results
- Better than or equal to the reference method – Accuracy validated by 3rd parties (AOAC-RIPTM)

Single-Step Enrichment
Less time spent on prep means cost savings for food manufacturers and third-party reference labs. Actero™ Elite Salmonella Enrichment medias single-step enrichment reduces “time-to-results” and minimizes possible errors, providing faster turnaround for product release decisions.

Robust List of Validated Matrices
These matrices make up some of the most commonly encountered in any food processing environment. Many matrices can interfere with PCR, preventing target amplification. Validated enrichment media can help control this inhibition, ensuring you get the pathogen detection information you need to make key food safety decisions.

| Catalog No. | Description | Quantity | |
| MED2030 | Actero™ Salmonella Enrichment Media | 2kg | |
| MED2024 | Actero™ MediaBox™ Salmonella | 5L | |
| MED2025 | Actero™ MediaBox™ Salmonella | 10L |
StatMedia Soluble Packets

StatMedia Soluble Packets
BAX® System MP Media in Convenient, Water-Soluble Packets
These easy-to-use packets provide the same performance as a powder but without the dust or ergonomic issues of handling bulk media. Other benefits include less hands-on time, less waste from expired batches of prepared broth, and reduced need for autoclaving. Packets are wrapped in a water-soluble film made from a polyvinyl alcohol resin. In warm water, the film dissolves within seconds. Because the soluble packets are gamma-irradiated, the prepared broth does not require autoclaving when used within 3-4 hours. For longer storage, broth can be autoclaved and then refrigerated for up to two weeks.
- Qty: 100 (33.75 g) packets
- Catalog Number: MED2016
Increased
Lab Productivity

Simplified
Workflow

Accurate
Results

24-Hour
Support

Dedicated
Applications Lab

| Catalog No. | D-Code | Description | Quantity | |
| MED2016 | N/A | StatMedia™ Soluble Packets | 100 |
Oxoid LEB Complete Media

Oxoid LEB Complete Media
Supplying optimal growing conditions for all Listeria species, Oxoid™ 24 Listeria Enrichment Broth (LEB) allows for the selective enrichment of Listeria monocytogenes and other Listeria spp. from food and environmental samples, enabling next-day results when using the BAX® System Genus Listeria 24E and Real-Time Listeria assays.
- Oxoid™ 24 LEB Complete Media
Increased
Lab Productivity

Simplified
Workflow

Accurate
Results

24-Hour
Support

Dedicated
Applications Lab

| Catalog No. | Description | Quantity | |
| MED2005 | Oxoid™ 24 LEB Complete Media | 2.5 kg | |
| MED2000 | Oxoid™ 24 LEB Buffer Supplement | 20 x 10 mL |
Hygiena Dehydrated Culture Media

Hygiena Dehydrated Culture Media
Buffered Peptone Water (BPW) is a non-selective pre-enrichment medium commonly used to help improve the recovery of injured Salmonella cells for the rapid detection of this pathogen in the food safety industry.
Research has demonstrated that BPW also can be an effective pre-enrichment medium for the recovery of Cronobacter species (E. sakazakii) from food and environmental samples.
Available in 500 g and 2.5 kg formats.
- Hygiena™ Dehydrated Culture Media (Buffered Peptone Water) 500 g; 2500 g; (yields about 25L and 125L, respectively)
Increased
Lab Productivity

Simplified
Workflow

Accurate
Results

24-Hour
Support

Dedicated
Applications Lab

| Catalog No. | Description | Quantity | |
| MED2011 | BPW | 500 g | |
| MED2010 | BPW | 2.5 kg |
BAX System MP Media

BAX System MP Media
A single selective enrichment broth optimized to detect E. coli and Salmonella with the BAX® System. The media provide nutrients and stable pH conditions to improve recovery from sponges and swabs used to sample food manufacturing environments.
The media enable next-day results for environmental samples tested with the BAX® System PCR Assay for E. coli and Salmonella (including the BAX® System X5).
Available as bulk powder (2.5 kg and 10 kg).
- BAX® System MP media – 2.5 kg (makes about 111 L)
BAX® System MP Media – 10 kg (makes about 444 L)
Increased
Lab Productivity

Simplified
Workflow

Accurate
Results

24-Hour
Support

Dedicated
Applications Lab

| Catalog No. | Description | Quantity | |
| MED2003 | BAX® System MP Media | 2.5 kg | |
| MED2029 | BAX® System MP | 10 kg |
BAX System Listeria Media

BAX System Listeria Media
A single selective enrichment broth optimized to detect Listeria with the BAX® System. The media provide nutrients and stable pH conditions to improve recovery from sponges and swabs used to sample food manufacturing environments.
The media enable next-day results for environmental samples tested with the BAX® System PCR Assay for Genus Listeria and Listeria monocytogenes (including the BAX® System X5).
Available as bulk powder (2.5 kg).
- BAX® System Listeria media – 2.5 kg (makes about 41 L)
Increased
Lab Productivity

Simplified
Workflow

Accurate
Results

24-Hour
Support

Dedicated
Applications Lab

| Catalog No. | Description | Quantity | |
| MED2002 | BAX® System Listeria Media | 2.5 kg |












