Karl Fischer titrators are designed for high accuracy, flexibility and reproducibility, allowing the user to obtain both accurate results and high-speed analysis. These titrators are designed to perform titrations for a variety of applications. Reports and methods can be transferred to a PC via a USB interface or saved to a USB storage device. Users can also print reports directly from these titrators using a standard parallel printer. An external monitor and keyboard may be attached for added versatility, as well as an analytical balance for automatic sample mass entry for titrations.
- Tester
- Portable Meters
- BenchTop Meters
- Titrators
- Photometers
- Refractometers
- Turbidimeters
- Thermometers
- Chemical Test Kits
- Magnetic Stirrer
- Monitors and Dataloggers
- Process Control
- Electrodes and Probes
- Solutions
- Reagents
- Accessories
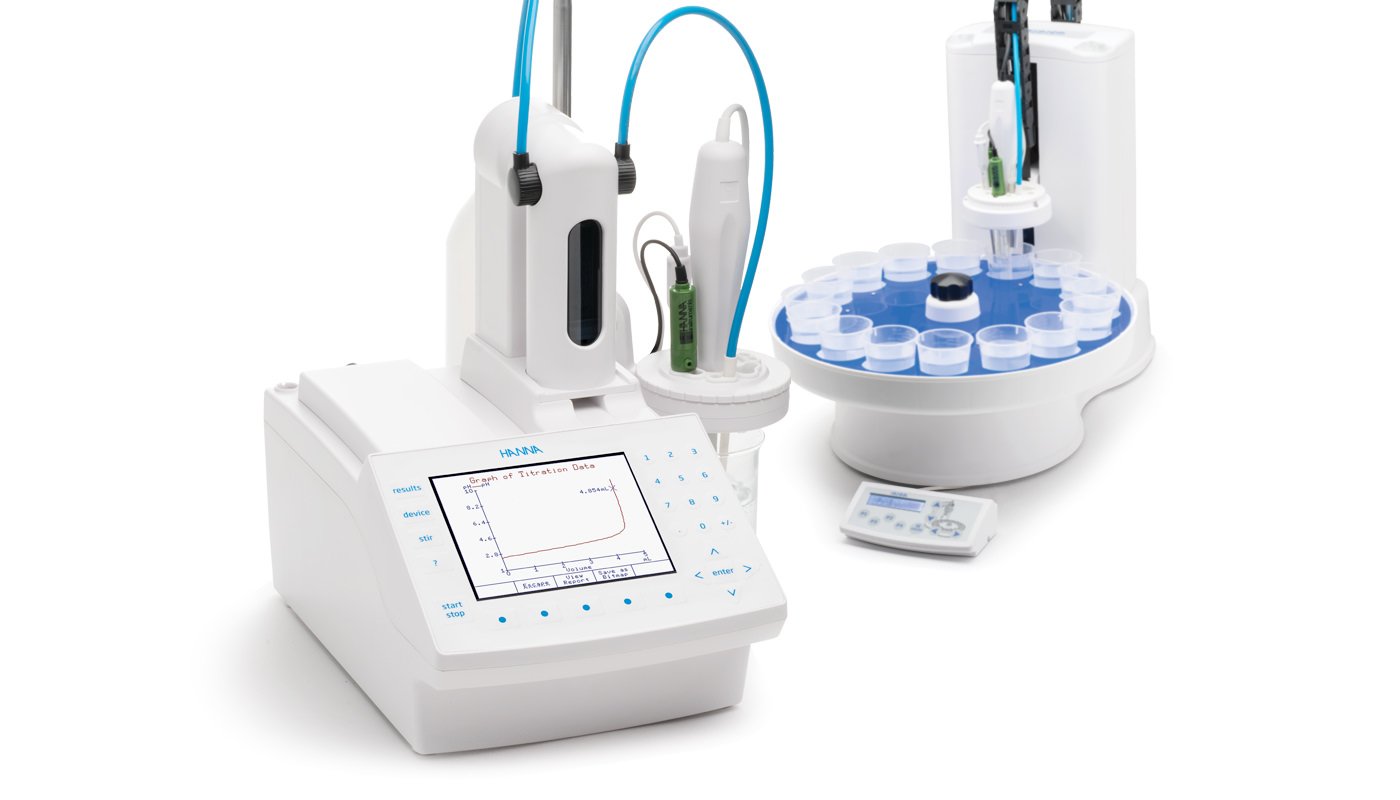
Titrators are designed to perform a wide variety of potentiometric titrations with high accuracy, flexibility, and reproducibility, allowing the user to obtain both accurate results and high-speed analysis. These titrators can perform fixed endpoint or equivalence point titrations and direct measurements by measuring the pH/mV and temperature of the sample. Reports and methods can be transferred to a PC via a USB interface, saved to a USB storage device, or printed directly from the titrator. An external keyboard can also be attached for added convenience.
Titration is used in analytical chemistry to determine the amount or concentration of a substance, known as the analyte. Titration is a quantitative measurement of an analyte in solution by its complete reaction with a reagent. In a titration, one reagent (the titrant) is slowly added to a solution containing the species being measured (the analyte). As it is added, a chemical reaction occurs between the titrant and analyte. The point at which the reaction is complete and an equivalent quantity of titrant and analyte are present (a stoichiometric equivalent) is called the equivalence point. This can be determined by a chemical indicator that is also present in the solution, or by a measurable physical change in the solution, like pH, electrode potential, conductivity, or light absorption (color). In practice, an abrupt change of this physical property signals the end of titration, called the endpoint.
The purpose of titration is to determine the quantity or concentration of an analyte with a known concentration and volume of a titrant. Titrations are based on chemical reactions which must fulfill four requirements:
- The reaction between the analyte and the titrant must occur quickly, without a secondary reaction
- The reaction must go to completion
- The reaction must have well-known stoichiometry (reaction ratio)
- Must have a convenient method of endpoint detection
Automatic titration is done with instrumentation that delivers the titrant, stops at the endpoint and calculates the concentration of the analyte automatically. Automatic titrators are best for accurate and repeatable results, as an electrochemical measurement is used to determine the endpoint as opposed to a subjective color indicator.
Analyses that can be performed by potentiometric automatic titrators include:
- Acid-base titrations
- Oxidation reduction titrations
- Complexometric titrations
- Precipitation titrations
- Non-aqueous titrations
- Argentometric titrations
- pH, ORP, and Ion selective measurements
Automatic Potentiometric Titrator - HI931
The HI931 Automatic Titrator is the answer to your dedicated titration needs. Fully customizable, the HI931 delivers accurate results and intuitive user experience, all in a compact package. Titrate for a variety of measurements at the push of a button including acids, bases, redox, and selective ions. No additional programming upgrades to purchase. The only things you need to start using the HI931 are a sensor and titrant.
- Small footprint so you can fully optimize your benchtop.
- Unmatched 40,000-step dosing pump for small volumes of titrant to help you achieve a very precise endpoint for greater consistency.
- Flexibility to store up to 100 methods.

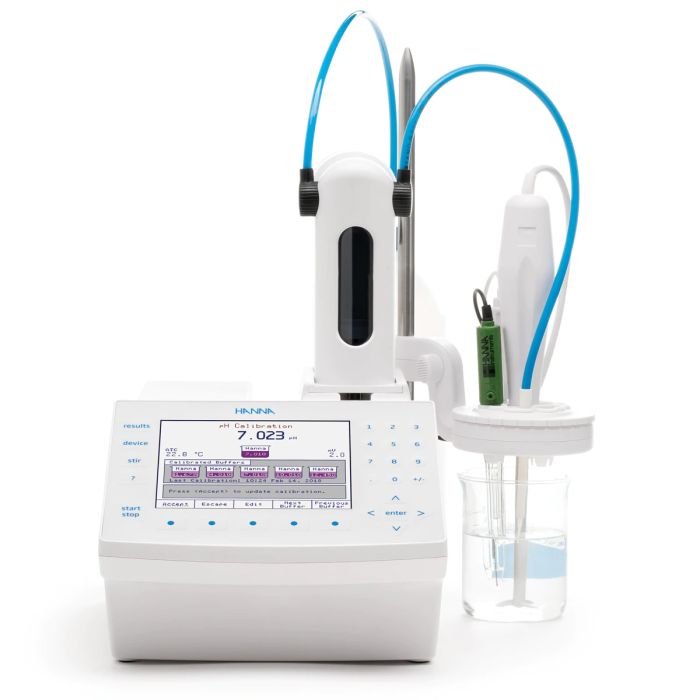
Automatic Potentiometric Titrator - HI932
The HI932 Automatic Titrator is the answer to your dedicated titration needs. Fully customizable, the HI932 delivers accurate results and intuitive user experience, all in a compact package. Titrate for a variety of measurements at the push of a button including acids, bases, redox, and selective ions. No additional programming upgrades to purchase. The only things you need to start using the HI932 are a sensor and titrant.
- Small footprint so you can fully optimize your benchtop.
- HI932 can be ordered with one or two analog boards for sequential linked titrations
- HI932 is compatible with HI922 autosampler for analyzing up to 18 samples at a time

HI901W Automatic Potentiometric Titrator for Wine
All-in-one titration solution made for wine.
The Wine Titrator is perfect for winemakers who need accurate results, ease-of-use, and the ability to expand the system as their analytical needs grow. It comes preloaded with methods for wine analysis, and with Hanna, you get the support you need to run them perfectly in your lab.
Get the most out of one device.
Titrate for a variety of measurements including pH, Total Acidity, Free & Total Sulfur Dioxide, Formol Number, Volatile Acids, Reducing Sugars, and many more.
Hanna’s Wine Titrator comes preloaded with methods for wine analysis, and with Hanna, you get the support you need to run them perfectly in your lab.
Titrator Dosing System
- Change reagents without worry.
Feel confident in your results.
Better design for increased precision.
Display & Navigation
- All of your important information is displayed.
Track your progress in real-time.
Explore titrator menus with ease.
Titrator Data & Reporting
- Highlight your most important information.
Safeguard the integrity of your data.
Move your information easily across devices.
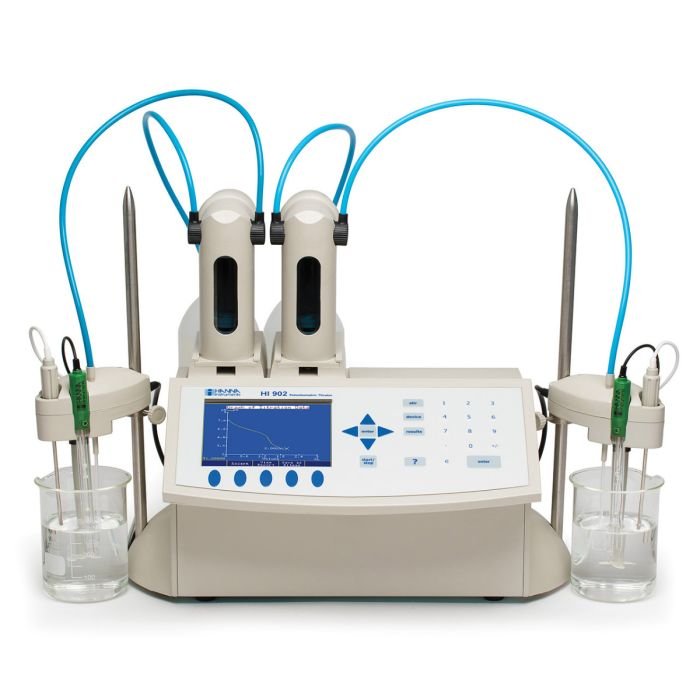
Automatic Potentiometric (pH/mV/ISE) Titration System - HI902C
The HI902C is an automatic titrator that complements our wide range of products dedicated to efficient and accurate laboratory analysis. The HI902C potentiometric titrator can perform acid/base, redox (ORP), complexometric, precipitation, non-aqueous, argentometric, and ion selective titrations, as well as back titrations and titre determinations. This powerful titrator automatically dispenses the titrant, detects the endpoint, and performs all necessary calculations and graphing. In addition to titration, the HI902C also operates as a fully functional pH, mV/ORP, and ion selective electrode (ISE) meter.
- Enhanced Security Options
- Dynamic Titrant Dosing
- Signal Stability Timing
- Equivalence Endpoint Detection
- Multiple Equivalence Point Detection
- Method Sequencing
- Small footprint, requires minimal bench space
- Casing made with strong, chemically resistant plastic
- Powerful built-in algorithms for termination criteria based on fixed mV endpoint or absolute/relative drift
- Titrant standardization and sample analysis averaging
- Minimized water vapor entry with the sealed solvent system
- Balance interface for automatic weighing
- Support for 100 titration methods
- User-customizable reports
- Clearly displayed warning and error messages

Coulometric Karl Fischer Titrator - HI934
For scientists and professionals who need exact water content determination from 1 ppm to 5%, our new generation of the Coulometric Karl Fischer Titrator is engineered to meet or exceed your technical requirements while delivering a lower cost of ownership. With cutting-edge features packed into a compact design, this titrator delivers accurate results and requires less space in the lab. Get accurate water content determination at the push of a button. Designed for the modern lab, this karl fischer titrator is the perfect fit for your testing environment.
- Compact design will save space in the lab without compromising your results.
- Durable heat and chemical resistant body.
- Sealed solvent system helps to keep water out of the system, while giving you minimal exposure to the reagents.

Volumetric Karl Fischer Titrator - HI933
For scientists and professionals who need exact water content determination from 0.01 to 100%, our new generation of the Volumetric Karl Fischer Titrator is engineered to meet or exceed your technical requirements while delivering a lower cost of ownership. With cutting-edge features packed into a compact design, this titrator delivers accurate results and requires less space in the lab. Get accurate water content determination at the push of a button. Designed for the modern lab, this karl fischer titrator is the perfect fit for your testing environment.
- Compact design will save space in the lab without compromising your results.
- Durable heat and chemical resistant body.
- Sealed solvent system helps to keep water out of the system, while giving you minimal exposure to the reagents.
Autosamplers are automated titration sample handling systems designed for use with the HI932 or HI902C Automatic Titration Systems respectively, making multiple sample titrations quick and easy.
With the Autosampler, up to 18 samples can be run consecutively. Hanna Autosamplers interface directly with the compatible titrator to access titration methods. Once a titration method is established, the user can fully customize the automation sequence of their samples for this method. Sample names and size can be customized or auto-filled with preset values. One beaker can be designated for storage purposes before and after titration sequences; up to three beakers per tray can be designated for an electrode rinse sequence, allowing for sufficient removal of solutions that are hard to clean between each sample titration. During each sample titration, the real-time progress is shown on the Automatic Titrators display. Finished sample results and graphs can be accessed during and after the titrations have finished.
Once the Autosampler sequence is complete, two reports are available for review: a sequence report featuring a table outlining each sample name, beaker position, sample size, and result for the tray, and a detailed titration report for each individual sample, including the graph of the titration data.

Autosampler for Potentiometric Titrator - HI921
The HI921 Autosampler is an automated titration sample handling system designed for use with the HI902C Automatic Titration System. This high quality system makes the titration of multiple samples quick and easy. The HI921 Autosampler interfaces directly with the HI902C to access titration methods, allowing up to 18 samples to be run consecutively.
The HI921 can utilize up to three peristaltic pumps for reagent addition, sample leveling, and waste aspiration. An included control panel allows for manual operation of the motors and pumps. The Autosampler also features a built-in magnetic stirrer or optional overhead stirrer, status indicator lights, USB interface with compatible barcode reader, and built-in RFID for each tray.
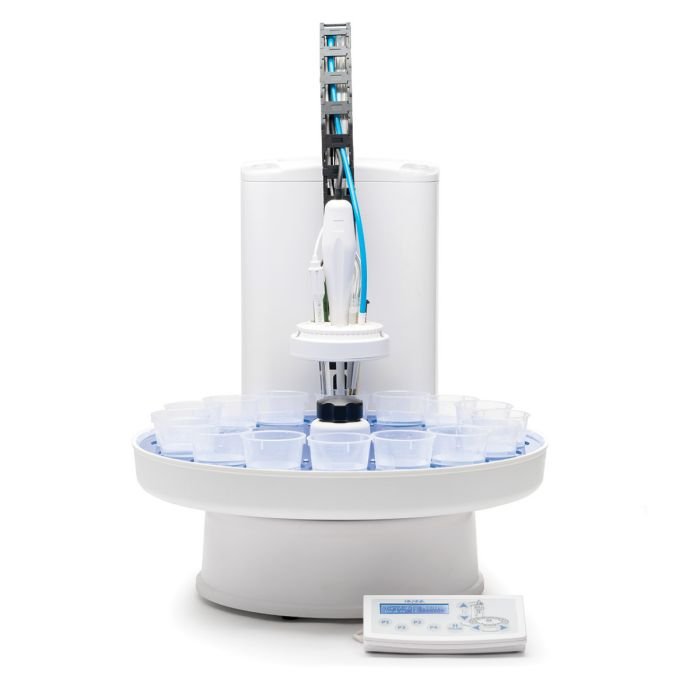
Autosampler for Potentiometric Titrator - HI922
The HI922 Autosampler is an automated titration sample handling system designed for use with the HI932 Automatic Titration System. This high quality system makes the titration of multiple samples quick and easy. The HI922 Autosampler interfaces directly with the HI932 to access titration methods, allowing up to 18 samples to be run consecutively.
The HI922 can utilize up to three peristaltic pumps for reagent addition, sample leveling, and waste aspiration. An included control panel allows for manual operation of the motors and pumps. The Autosampler also features a built-in magnetic stirrer or optional overhead stirrer, status indicator lights, USB interface with compatible barcode reader, and built-in RFID for each tray.

Mini Titrator for Measuring Sulfur Dioxide in Wine - HI84500
The HI84500 is a fast, accurate, and affordable automatic mini titrator designed for testing free or total sulfur dioxide (SO2) levels in wine, juice, and must. Based on the Ripper Method, this mini titrator uses an optimized, preprogrammed method of analysis with a powerful algorithm that determines the completion of the titration reaction by the use of a specialized ORP electrode.
The HI84500 incorporates a precision piston style dosing pump that adjusts the volume of dosing dynamically based on the voltage changed. This dosing system reduces the amount of time required for the titration while providing for a highly accurate determination of the amount of titrant used.
This mini titrator is supplied complete with all the materials necessary to perform low and high range measurement of SO2. All chemicals are premixed and prepackaged including standardized titrants, reagents and pump calibration solution. There is no need for volumetric glassware or analytical balances.
- Piston driven pump for accurate dynamic dosing
- Complete with ORP electrode with CPS™ Technology
- Pre-standardized titrant and pre-measured reagents

Mini-Titrator for Measuring Titratable Acidity in Wine - HI84502
The HI84502 is a simple, fast, and affordable automatic mini titrator designed for testing total acidity levels in wine. Based on an acid-base titration method, this mini titrator uses an optimized preprogrammed method of analysis with a powerful algorithm that determines the completion of the titration reaction by the use of a specialized wine pH electrode.
Analysis for titratable acidity with the HI84502 is not selective for acids of similar strength. In this method, a base of known concentration is used to understand the overall acid content of a sample and express it as the main acid present in wine, tartaric acid.
The HI84502 incorporates a precision piston style dosing pump that adjusts the volume of dosing dynamically based on the voltage changed. This dosing system reduces the amount of time required for the titration while providing for a highly accurate determination of the amount of titrant used.
This mini titrator is supplied complete with all the materials necessary to perform low and high range measurements of total acidity. All chemicals are premixed and prepackaged including standardized titrants, reagents and pump calibration solution. There is no need for volumetric glassware or analytical balances.

Mini Titrator for Measuring Formol Number in Wine and Fruit Juice - HI84533
The HI84533 is a simple, fast, and affordable automatic mini titrator designed for testing formol number in wines or fruit juices. Based on an acid-base titration method, this mini titrator uses an optimized pre-programmed method of analysis with a powerful algorithm that determines the completion of the titration reaction by the use of a glass body pH electrode.
The HI84533 incorporates a precision piston style dosing pump that adjusts the volume of dosing dynamically based on the voltage change. This dosing system reduces the amount of time required for the titration while providing for a highly accurate determination of the amount of titrant used.
This mini titrator is supplied complete with all the materials necessary to perform low and high range formol measurements. All chemicals are premixed and prepackaged including standardized titrants, reagents and pump calibration solution. There is no need for volumetric glassware or analytical balances.

Mini Titrator for Measuring Titratable Acidity in Dairy Products - HI84529
The HI84529 is an easy to use, fast and affordable automatic mini titrator designed for testing titratable acidity levels in dairy products. Based on an acid-base titration method, this mini titrator uses an optimized pre-programmed method of analysis with a powerful algorithm that determines the completion of the titration reaction by the use of a specialized foodcare pH electrode.
The HI84529 incorporates a precision piston style dosing pump that adjusts the volume of dosing dynamically based on the voltage change. This dosing system reduces the amount of time required for the titration while providing for a highly accurate determination of the amount of titrant used.
This mini titrator is supplied complete with all the materials necessary to perform low and high range measurements of titratable acidity. All chemicals are premixed and prepackaged including standardized titrants, reagents and pump calibration solution. There is no need for volumetric glassware or analytical balances.
- Piston driven pump for accurate dynamic dosing
- Complete with anti-clogging reference electrode
- Pre-standardized titrant and pre-measured reagents

Mini Titrator for Measuring Titratable Alkalinity in Water and Wastewater - HI84531
The HI84531 is an easy to use, fast and affordable automatic mini titrator designed for testing titratable alkalinity levels in water. Based on an acid base titration method, this mini titrator uses an optimized pre-programmed method of analysis with a powerful algorithm that determines the completion of the titration reaction by the use of a glass body pH electrode.
The HI84531 incorporates a precision piston style dosing pump that adjusts the volume of dosing dynamically based on the voltage change. This dosing system reduces the amount of time required for the titration while providing for a highly accurate determination of the amount of titrant used.
This mini titrator is supplied complete with all the materials necessary to perform low and high range measurements of alkalinity in water. All chemicals are premixed and prepackaged including standardized titrants, reagents and pump calibration solution. There is no need for volumetric glassware or analytical balances.
- Piston driven pump for accurate dynamic dosing
- Complete with glass double junction pH electrode
- Pre-standardized titrant and pre-measured reagents

Mini Titrator for Measuring Titratable Acidity in Water - HI84530
The HI84530 is an easy to use, fast and affordable, method specific titrator designed for testing titratable acidity levels in water. Based on an acid-base titration method, this mini titrator uses an optimized pre-programmed method of analysis with a powerful algorithm that determines the completion of the titration reaction potentiometrically with a glass body pH electrode.
The HI84530 incorporates a precision piston style dosing pump that adjusts the volume of dosing dynamically based on the voltage change. This type of dosing system reduces the amount of time required for the titration while providing for a highly accurate determination of the amount of titrant used.
This mini titrator is supplied complete with all the materials necessary to perform low and high range measurements of acidity in water. All chemicals are premixed and prepackaged including standardized titrants, reagents and pump calibration solution. There is no need for volumetric glassware or analytical balances.
- Piston driven pump for accurate dynamic dosing
- Pre-standardized titrant and pre-measured reagents
- Benchtop use as a pH/mV meter