Test Kit for SARS-CoV-2
LIFE SCIENCE & MOLECULAR BIOLOGY PRODUCTS AND CONSUMABLES
- Antibody Detection Kit (ELISA)
- RT-PCR Kit
- Truss System

COVID-19: Antibody Detection Kit (ELISA)
Antibody ELISA test improves the sensitivity of early diagnosis and identify the patients during periods of incubation or early infection or asymptomatic infection.
SARS-CoV-2 Virus IgG Antibody Detection Kit (ELISA)
SARS-CoV-2 Virus IgM Antibody Detection Kit (ELISA)
BGI has developed SARS-CoV-2 IgM and IgG antibody detection kit. SARS-CoV-2 ELISA kit uses the principle of capture method (ELISA) and indirect capture method (ELISA) to detect SARS-CoV-2 IgM and IgG antibody in human serum or plasma. The kits are CE marked. Detection of IgM antibodies tends to indicate recent exposure to SARS-CoV-2, whereas the detection of COVID-19 IgG antibodies indicates virus exposure for a relatively long period.
Antibody ELISA test could not only be used as a supplementary test for suspect with RT-PCR negative cases for COVID-19, but also as a supplementary detection indicator in conjunction with nucleic acid detection in the diagnosis of suspected cases. Antibody ELISA test improves the sensitivity of early diagnosis and identify the patients during periods of incubation or early infection or asymptomatic infection.
- High Specificity The clinical specificity of IgM ELISA kit is 96.76%, and that of IgG ELISA kit is 98.38%.
- High Sensitivity The sensitivity of IgG ELISA kit is 99.16%( more than 20 days), The sensitivity of IgM ELISA kit is 98.06%(10-19 days). And the sensitivity of total IgG and IgM is more than 98.71%
- Satisfactorily homogeneous High reproducibility, the coefficient of variation (CV) is no larger than 10%,
- High Throughput With automated ELISA system, over 20,000 assays can be processed in one day.
- Convenient No space separation required by majority of PCR kit, suitable for high throughput test.
- Cost-effective Cost-effective systems and reagents; less time and effort are required for operation
Suitable to:
1. Validate the SARS-CoV-2 infection on suspected cases
2. Find potential asymptomatic SARS-CoV-2 carriers
3. Identify the immunized population
4. Determine if the patients can be discharged from the hospital or have a second infection
5. Test the efficacy of vaccines
2019-nCoV: Real-Time Fluorescent RT-PCR kit
With the high specificity, sensitivity and rapid response of the RT-PCR Kit, it can effectively assist the diagnosis of disease and improve the diagnosis efficiency.
RT-PCR Test Kit
BGI’s Real-Time Fluorescent RT-PCR kit for detecting 2019-nCoV(SARS-CoV-2) is a qualitative in vitro nucleic acid amplification assay used to detect SARS-CoV-2 using reverse transcription PCR from throat swab and Bronchoalveolar Lavage Fluid(BALF) samples. The kit is widely used for rapid detection and outbreak control of COVID-19 in China. BGI is distributing its RT-PCR kits to more than 180 countries and regions around the world.
With its high specificity, sensitivity and rapid response, it can effectively assist the diagnosis of disease and improve the diagnosis efficiency.
The kits are internationally available, and are currently being distributed to more than 50 countries and regions worldwide. Each kit provides reagents sufficient for 50 reactions.
Product Features
- NMPA Certified / CE Marked / FDA Authorized / PMDA Approved
- Results issued in 3 hours
- One Step-Reaction
- High Sensitivity and Specificity
LOD of 100 copies/mL
No cross-reactivity with human genome and other pathogens including 54 pathogens, such as human coronavirus OC43, 229E, HKU1 and NL63(HCoV-OC43, HCoV-229E, HCoV-HKU1, HCoV-NL63) etc.
Whole-Process Quality Control
An internal control was set to monitor the laboratory procedures including nucleic acid extraction, Revert transcription and amplification in each reaction.
- Compatible to Universal Real time PCR system
- Over 1.2 Million Tests kits have been produced
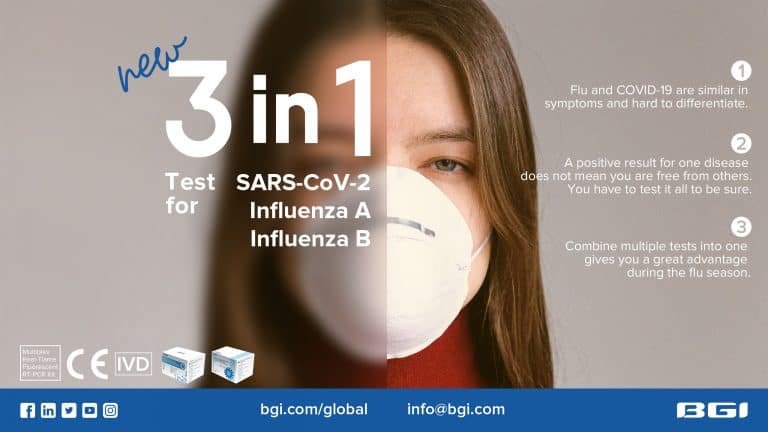
New 3-in-1 Test For SARS-CoV-2, Influenza A and Influenza B
BGI’s new CE certified 3-in-1 test For SARS-CoV-2, Influenza A and Influenza B provide clinicians and doctors with a reliable single-panel test to help quickly and accurately diagnose patients.
New 3-in-1 Test For SARS-CoV-2, Influenza A and Influenza B
COVID-19 shares many symptoms with the flu and this fact will, unfortunately, exacerbate the many existing challenges in diagnosing patients with non-specific respiratory symptoms during flu season.
BGI’s new CE certified 3-in-1 test For SARS-CoV-2, Influenza A and Influenza B provide clinicians and doctors with a reliable single-panel test to help quickly and accurately diagnose patients.
- Flu and COVID-19 are similar in symptoms and hard to differentiate.
- A positive result for one disease does not mean you are free from others. You have to test it all to be sure.
- Combine multiple tests into one give you a great advantage during the flu season.
- CE Marked
The kits are internationally available and can be distributed to several countries and regions worldwide.
![]()
![]()